Chapter 6: Search for Order
chemical symbols | periodic
table | group names
periodic
table (on a separate page)
Chemical symbols
Each chemical element is represented by a symbol. Sometimes it's a single
letter (for example H for hydrogen, C for carbon, etc), while sometimes
it's two letters (for example He for helium, Li for lithium, etc). The
first (or only) letter is always a capital. The second letter (if there
is one) is always a small letter.
These chemical symbols are combined to make the chemical formulae
(the plural of formula, also written formulas) for materials in the same
way the element atoms are combined to form molecules. For example, water
is H2O, which means that two atoms of hydrogen combine with
one atom of oxygen to make a single water molecule.
Some elements combine with themselves to make molecules of a single element.
For example, hydrogen and oxygen gases have the formulae H2
and O2.
Chemical equations are used to describe how substances are made.
For example, water is made from hydrogen and oxygen:
2H2 + O2 → 2H2O +
heat
(That's an arrow in the middle.) The big 2 in front of the H2 and H2O formulae applies to the whole molecule after the 2. That means on the left there are 2 molecules of H2 (4 hydrogen atoms) and on the right 2 molecules of H2O (4 hydrogen atoms, 2 oxygen atoms). Often the "heat" isn't
mentioned in the formula.
The periodic table, or periodic table of
the chemical elements
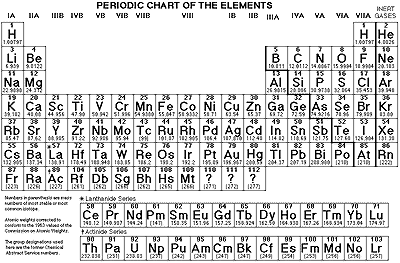
Click for larger version.
The word "periodic" means recurring. This means that
each column, or group (sometimes also called a family) of the periodic
table contains elements which have similar chemical and physical properties.
For example, the money metals from chapter 2 are all from group 11 of
the periodic table. They are all quite inert chemically, and all have
similar physical properties, such as electrical conductivity. (The most electrically conductive
of all metals are silver, copper and gold in that order.)
As we go on through the elements there are more elements in the rows
(or periods) of the periodic table, so the first row has only two
elements, then the next two rows have 10 elements each, then a couple
of rows with 18, and a couple with 32. (About there we run out because
all the last ones are man-made in particle accelerators and get more
and more difficult to make, and are radioactive, so decay into other elements.)
The increasing number of elements per period is because of the way the
element's electrons fill up electron shells. We don't need to worry about
that too much, but it's interesting to note the following:
-
If an element's outermost electron shell is full the element is very
stable. These elements together form a group called the noble gases
on the far right side of the periodic table.
-
If an element's outermost electron shell is nearly empty then the element is very reactive. This is why the first two groups are so reactive - they have only
one or two electrons to lose from their outer shell to get a full outer shell.
-
If an element's outermost electron shell is nearly full then the element is very reactive. This is why the two groups just to the left of the noble gases are so reactive - they have to gain only
one or two electrons to fill their outer shell.
In the periodic table metals are on the left, and non-metals are on the
right, except hydrogen, which is only metallic at high temperature and
pressure. We can further divide them up into metals,
poor
metals, metalloids,
and non-metals.)
All the elements are solids except for two elements which are liquid
at room temperature (bromine and mercury), and a few which are gases (hydrogen,
nitrogen, oxygen, fluorine, chlorine, and the noble gases of group 18).
Two other elements (galium and caesium) have melting points under 30 °C.
Uranium, element 92, is the highest number to occur naturally. 118 elements
have so far been found or made.
The lanthanides and the actinides are normally placed below the table
simply so that the table doesn't get impractically
wide like the extended periodic table.
The first useful periodic table was created in 1869 by a Russian chemist
named Dimitri Mendeleev. His table and some other very interesting arrangements
of the elements are shown
here.
Group names
Some of the groups have been given special names.
Group 1: Alkali metals (except hydrogen which is a non-metal).
Group 2: Alkaline earth metals.
Groups 3 to 12: Transition metals, which includes group 11, unofficially
called the money metals or coinage metals, or sometimes the noble metals.
(Note: In chemistry a "noble metal" is one which is resistant
to corrosion, and includes gold, silver, platinum, tantalum, etc, although
physics has a narrower definition which means that only copper, silver
and gold are noble metals.)
Group 13: Boron group.
Group 14: Carbon group.
Group 15: Nitrogen group. Also unofficially called the pnicogens
or pnictogens.
Group 16: Chalcogens (with the "ch" pronounced with
a hard "c" as in "chemistry") - oxygen, sulphur, etc
Group 17: Halogens - fluorine, chlorine, etc.
Group 18: Noble gases or inert gases - helium, neon, etc.
|

