Chapter
9: Compounds by Electrical Attraction
electrical attraction | sodium | chlorine | sodium chloride | seawater | potassium chloride | potassium fluoride
Electrical attraction
Atoms want to gain or lose electrons to fill their outermost shell, like
the noble gases. Elements on the left of the periodic table want to lose
electrons while those on the right (not counting the noble gases) want
to lose electrons. Because electrons are negatively charged, atoms that
lose an electron will have a positive charge and atoms that gain an electron
will have a negative charge. Because opposites attract, like a match made
in heaven atoms of elements from the left of the table will react with
those on the right and donate an electron to them, thereby filling the
outermost electron shell of both atoms.
For example, sodium (Na) will want to lose an electron to become Na+ while chlorine (Cl) will want to gain an electron to become Cl-.
An atom that has a charge like this is called an ion. The two ions combine
to form sodium chloride, otherwise known as table
salt.
Na + Cl → NaCl

Sodium
Sodium is a soft silvery metal, symbol Na (from Latin "natrium"). atomic
number 11. First isolated in 1807 by Sir Humphry Davy through the electrolysis
of caustic soda.
It is very reactive and needs to be kept under mineral oil or kerosene
so that it doesn't react with the air. Sodium has a low melting point,
just 97.72 °C. When sodium burns in air it also melts and runs everywhere
burning as it does. (A bit like napalm.) Sodium is more reactive than
magnesium and if sodium catches fire it's very hard to put out. Very few
types of fire extinguishers will work on it.
It is very light weight, just a bit lighter than water. Because of this
(and the production of hydrogen), when placed in water "small pea-sized
pieces will swim around the surface of the water until they are consumed
by it, whereas large pieces will explode" (Wikipedia).

Ronald Reagan advertising borax.
|
Sodium is a component of sodium chloride or salt (NaCl), soda ash
(Na2CO3), baking soda (NaHCO3),
caustic soda (NaOH), Chile saltpetre (NaNO3) and borax
(Na2B4O7·10H2O). |
 Low
pressure sodium vapour lamps are a characteristic yellow-orange
colour, which comes from their strong double spectral line in the
yellow-orange part of the spectrum. Wikipedia: "Sodium is relatively
abundant in stars and the D spectral lines of this element are among
the most prominent in star light. Sodium makes up about 2.6% by
weight of the Earth's crust making it the fourth most abundant element
overall and the most abundant alkali metal." Low
pressure sodium vapour lamps are a characteristic yellow-orange
colour, which comes from their strong double spectral line in the
yellow-orange part of the spectrum. Wikipedia: "Sodium is relatively
abundant in stars and the D spectral lines of this element are among
the most prominent in star light. Sodium makes up about 2.6% by
weight of the Earth's crust making it the fourth most abundant element
overall and the most abundant alkali metal."
Wikipedia: "Sodium ions are necessary for regulation of blood
and body fluids, transmission of nerve impulses, heart activity,
and certain metabolic functions." |
Chlorine
Chlorine is a pale green gas, symbol Cl, atomic number 17.
Gaseous chlorine is Cl2 and is about 2.5 times heavier than
air.
Chlorine is the third most reactive element (after fluorine and oxygen)
and will combine easily with almost all other elements. It is very toxic
and has a characteristic smell. Some of its uses:
- Disinfecting.
- Water purification.
- Sanitising swimming pools. (Non-commercial swimming pools use sodium
hypochlorite rather than chlorine gas.) For more information see the
Aqualab page Chlorine Shock Treatment.
- Bleaching.
- Chemical weapon (since World War I).
- Plastics.
Sodium chloride
Both sodium and chlorine are poisonous individually, but combine to make
sodium chloride, or table salt.
"Breaking up is hard to do." Because both sodium and
chlorine are very reactive, their product is very stable. It's very hard
to pull the sodium and chlorine atoms apart again. One way to do it would
be to heat salt with an even more reactive substance. Only oxygen and
fluorine are more reactive than chlorine and using one of these would
still leave the sodium in the resulting molecule.
2NaCl + Fl2 → 2NaFl + Cl2
4NaCl + O2 → 2Na2O + 2Cl2
Another way is to use electrolysis, although that requires that the NaCl
be dissolved in water or melted. Dissolving salt in water then passing
an electric current through it gives the equation:
2NaCl + 2H2O → Cl2 + H2 + 2NaOH
The last product, sodium hydroxide, is also known as caustic soda or
lye. It is a strongly caustic base (the opposite to an acid).
Melting salt is difficult because of its high melting temperature of
801 °C. However, mixing it with calcium chloride reduces its melting
point to something less than 700 °C.
2NaCl + energy → 2Na + Cl2
People need salt to live. Because of this and its rarity in the ancient
world, salt used to be very valuable. A Roman soldier would have been
incredulous to see modern people spreading salt on roads to melt snow
- a purpose which uses just over half the world's salt production. Even
more recently salt's value has been seen, such as in 1930 when Mohandas
Karamchand Gandhi (aka Mahatma Gandhi) organised protests in which "thousands
of Indians went to the sea to illegally produce their own salt in protest
of the British tax on salt."
In New Zealand the Adequate Intake (AI) figure for salt is just 1.2-2.3
g per day, or a quarter to half a teaspoon full. With a single salt sachet
from a fast food restaurant you almost hit the New Zealand Upper Limit
(UL) of 5.8 g. Too much salt gives us high blood pressure due to the sodium,
but too little will result in our muscles cramping up and many other problems.
It is essential for life.
Halite is the name for salt when it's found as a naturally occuring
mineral. Also called rock salt.
Saltwater
swimming pools use salt and electrolysis instead of adding direct-acting
chlorine chemicals. Ordinary salt is added to the pool water to bring
it to a salinity of 3-6 g/L (seawater is 35 g/L) then electricity is passed
through it in a chlorinator cell to make chlorine, which sanitises the
water.
Sumo wrestlers throw salt before their matches.

Seawater
The solubility of NaCl in water is roughly 360 g/L for 25 °C water.
Seawater on average has just under a tenth of that, with salinity of
35 g/L. The saltiness in seawater comes almost completely from NaCl. Seawater
should not be drunk, as the body uses more water to process the seawater
(and remove all the minerals) than it gets from it.
The Red Sea is the world's saltiest sea, and has a salinity of 36-38
g/L.
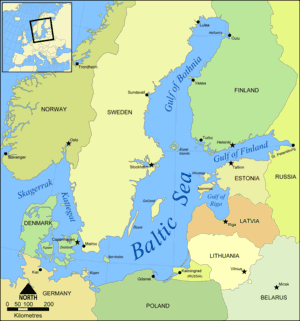 At
the north of the Baltic Sea, in the Gulf of Bothnia the salinity
can be as low as 1g/L (0.1%). This is basically because the inflowing
water pushes the seawater out, and floats on top of it. At
the north of the Baltic Sea, in the Gulf of Bothnia the salinity
can be as low as 1g/L (0.1%). This is basically because the inflowing
water pushes the seawater out, and floats on top of it.
It's said that the water is so fresh the salt can't be tasted in
it.
Experiment: About.com
claims humans have a salt taste limit of 3.5g/L. Is this right?
Because salt lowers the freezing point of water, as a consequence
of its low salt content the Gulf of Bothnia is frozen for five months
of the year. |
Elemental composition of Earth's ocean water (by mass)
| Element |
Percent |
| Oxygen |
87.7 |
| Hydrogen |
10.8 |
| Chlorine |
1.9 |
| Sodium |
1.05 |
| Magnesium |
0.1350 |
| Sulphur |
0.0885 |
| Calcium |
0.04 |
| Potassium |
0.0380 |
| Bromine |
0.0065 |
| Carbon |
0.0026 |
(Wait a mo - the total is 101.6% ???) |
|
 The
Dead Sea, a saltwater lake, has a salinity of 300g/L (30%) or 8.6
times normal seawater, which makes it very easy to float in the
Dead Sea. Wikipedia: "The mineral content of the Dead Sea is
significantly different from that of ocean water, consisting of
approximately 53% magnesium chloride, 37% potassium chloride and
8% sodium chloride (common salt) with the remainder comprised of
various trace elements." The
Dead Sea, a saltwater lake, has a salinity of 300g/L (30%) or 8.6
times normal seawater, which makes it very easy to float in the
Dead Sea. Wikipedia: "The mineral content of the Dead Sea is
significantly different from that of ocean water, consisting of
approximately 53% magnesium chloride, 37% potassium chloride and
8% sodium chloride (common salt) with the remainder comprised of
various trace elements."
Click for larger versions.

|
Hong Kong uses seawater for flushing its toilets.
Sodium chloride vs potassium chloride
Potassium chloride (KCl) is sometimes used as a replacement for table
salt because potassium doesn't cause high blood pressure like sodium does,
but it is often mixed with NaCl when this is done because KCl doesn't
taste as good (it's not as salty and a little bitter).
The solubility of KCl in water is the same as for NaCl, roughly 360 g/L
for 25 °C water.
Both NaCl and KCl are toxic when too much is eaten - 2.5 g salt per kg
of body weight, or about 100 g for the average 40kg 11-12 year old. However,
before this happens it's quite likely that you will have thrown up. Even
as late as the 1970s salt was used in hospitals to make children throw
up.
Wikipedia says "a massive overdose of intravenous potassium chloride
is used to stop the heart in execution by lethal injection."
Potassium fluoride
The chemical compound potassium fluoride (KF) is an alkali metal halide
that occurs naturally as the rare mineral carobbiite. Potassium fluoride
is used for etching glass and also as a preservative and insecticide.
Potassium fluoride is very hazardous to humans.
|

