Chapter 4: Gases in the Air
release by humans | hydrogen
| oxygen | carbon dioxide
| nitrogen | links
Release by humans
Humans release all the gases featured in this lesson. From Wikipedia:
The average human releases 0.5 to 1.5 litres of flatus a day by
flatulating 12 to 25 times. The primary constituents of flatulence are
the non-odorous gases nitrogen (ingested), carbon
dioxide (produced by aerobic microbes or ingested), and hydrogen
(produced by some microbes and consumed by others), as well as lesser
amounts of oxygen (ingested) and methane (produced
by anaerobic microbes). Odours result from trace amounts of other components
(often containing sulphur).
Serious stuff. Funny how it's the smallest portion (the sulphur-containing
smelly components) that we take most notice of.
Hydrogen
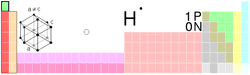 Hydrogen
is a non-metal* element, symbol H, atomic number 1. Hydrogen
is a non-metal* element, symbol H, atomic number 1.
* At extremely high pressures at the same time as very high temperatures
(~3,000 K) it changes to metallic hydrogen. Metallic hydrogen is believed
to be present in tremendous amounts in the gravitationally compressed
interiors of Jupiter and Saturn.
Hydrogen makes up 75% of the universe by mass, or 90% by number of atoms,
but only about 1 ppm in the atmosphere (abut 1 cubic centimetre hydrogen
per cubic metre of air - that's a cube of hydrogen 1 cm on each side in
a cube of air 1 m across). Because it's so light it easily escapes to
space.
Hydrogen gas is H2 (each molecule of hydrogen gas has two
atoms of hydrogen in it) and is easily made by (for example):
- Electrolysis of water.
- Cracking of methane (CH4).
- Reacting a metal with hydrochloric acid (HCl). Using sodium (Na) would
give H2 gas and table salt (NaCl).
- Mixing aluminium and chlorine bleach (don't try at home).
- Etc.
Hydrogen burns to make water, H2O (in gaseous form).
2H2 + O2 → 2H2O +
heat
Hydrogen-oxygen flames are nearly invisible to the naked eye, which is
why space rocket exhaust is pretty transparent. The space shuttle's main
engines run on hydrogen and so have almost transparent exhaust but the
shuttle also has solid-fuel booster rockets on the sides, which are basically
big fireworks. Because hydrogen burns so easily (in concentrations as
little as 4% in air) care must be taken when using it for combustion.
Oxygen
 Oxygen
is a non-metal element, symbol O, atomic number 8. Oxygen
is a non-metal element, symbol O, atomic number 8.
Oxygen gas is O2 and forms about 21% of our atmosphere. While
oxygen is very reactive, and oxygen can combine with almost all other
elements, it often needs heat applied to do so. Otherwise, reactions take
place in slow motion, like an apple slice going brown, or paper yellowing
with age.
In a pure oxygen environment fires can burn out of control, but if the
supplies of both oxygen and fuel are controlled, useful things like rockets
and oxyacetylene torches can be made. Acetylene burns at 3,200 to 3,300
°C (error in text).
Ozone (O3) is a form of oxygen found throughout the atmosphere.
In the upper atmosphere it's made by high energy electromagnetic radiation,
and helps to block UV light. At ground-level ozone it's made by electric
discharges (eg, electric motors, photocopiers, etc) and is regarded as
a pollutant. It's one of the most toxic inorganic compounds known (inorganic
means it doesn't contain either carbon or hydrogen) and is useful for
purifying air and water (including swimming pools).
Ozone can be smelled at just 15 ppb (part per billion), or 15 cubic millimetres
per cubic metre, and hence its name, from the Greek word for "smell".
(This is three times the concentration of just-detectable H2S.)
Liquid O2 and solid O2 have a light blue colour.
Liquid O2 is usually obtained by the fractional distillation
of liquid air. Liquid and solid O3 are dark blue.
Although ozone is a pollutant at ground-level
it's produced by devices described as "air purifiers" and "negative
ion generators". Often many of these devices are not intended to
make ozone (some are designed specifically to make it) they all make at
least some ozone.

The photo above is one is for a car, available online for about $11 including
shipping. It has two reactions on the packaging for the oxidation of two
smelly gases, which smell of rotten eggs and rotten cabbage respectively.
I'll leave it up to the reader to figure out where they might come from
in a car.
2H2S + O3 → H2O
+ SO2
CH3SH + O3 → CH3OH
+ SO2
Perhaps it should be stated that hydrogen sulphide (H2S), methanethiol
(methyl mercaptain, CH3SH), ozone (O3), methanol
(CH3OH) and sulphur dioxide (SO2) are all toxic.
However the methanol would be further oxidised thus:
CH3OH + O3 → CO2
+ 2H2O
Ozone is highly toxic. See Wikipedia air
ioniser and ozone
generator for some cautions when considering the use of one of these
devices. Being a small and inexpensive device it will use the coronal
discharge method of ozone production. When used in air (instead of pure
oxygen) as well as ozone it produces nitrogen oxides, which are also
toxic (see under Nitrogen for more information).
N2 + O2 → 2 NO
N2 + 2 O2 → 2 NO2
Because liquid oxygen is more concentrated than gaseous oxygen it is
a much better oxidiser and so is used for rocket propulsion, normally
with liquid hydrogen. It was also used by George Goble for his liquid
oxygen barbeque, for which he won the 1996 Ig Nobel award for chemistry.
He explains how things went for "lighting of the grill with 3
gallons (11.3 L) of liquid oxygen. Started with 60 lbs (27 kg) of charcoal,
and burnt up 40 lbs (18 kg) of it in 3 seconds. Result is a grill ready
to cook in about 3 seconds, and all the old grease, etc burned off. Don't
try this at home." He has also found that a charcoal briquette
soaked in liquid oxygen will explode with about the energy of a stick
of dynamite.
A recently discovered form of oxygen, tetraoxygen (O4),
is a deep red solid that is created by pressurising O2 to the
order of 20 GPa (about 200,000 atmospheres). Its properties are being
studied for use in rocket propulsion and similar applications, as it is
a much more powerful oxidiser than either O2 or O3.
Carbon dioxide
Carbon
dioxide is a compound, symbol CO2.
CO2 content in fresh air varies between 0.03% (300 ppm) to
0.06% (600 ppm) depending on location and in exhaled air is about 4.5%.
Breathing high concentrations of carbon dioxide is painful because the
carbon dioxide dissolves in the mucus lining of the lungs giving carbonic
acid. CO2 is not poisonous but it is suffocating - we can't
live on it.
Carbon dioxide is a gas at room temperature, and forms a solid known
as dry ice at -78 °C. When dry ice warms up, instead of melting it
sublimates - it turns straight into a gas. CO2 can be a liquid
at about five atmospheres and more, starting at -57 °C. CO2
gas is heavier than air, while dry ice is heavier than water.
CO2 is made when carbon combines with oxygen by combustion,
respiration, etc. Plants use CO2 to form carbohydrates, while
people use CO2 to make soft drinks, to power air guns, baking
(where the CO2 is made by yeast or baking soda/sodium
bicarbonate) and in some fire extinquishers.
In the 1750s the Scottish physician Joseph Black used limestone (calcium
carbonate, CaCO3) to make a gas he termed "fixed air"
which was denser than air and did not support either flame or animal life.
He also worked out that the gas is produced by animal respiration and
microbial fermentation.
In 1772, Joseph Priestley used carbon dioxide produced from the action
of sulphuric acid on limestone to prepare soda water, the first known instance
of an artificially carbonated drink.
Incomplete combustion of carbon results in carbon monoxide (CO),
which is odourless and very toxic because it combines with haemoglobin
in the blood preventing it from carrying oxygen around the body. The main
(common) source of CO is car exhaust, although catalytic converters reduce the carbon monoxide in car exhaust gases considerably.
Nitrogen
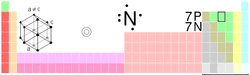 Nitrogen
is a non-metal element, symbol N, atomic number 7. Nitrogen
is a non-metal element, symbol N, atomic number 7.
Nitrogen gas is N2 and is the most common gas in the atmosphere,
making up 78% of it. Nitrogen is a very reactive element but N2
is very stable (it's joined by a triple bond), making it quite inert.
Nitrogen was discovered in 1772 by Daniel Rutherford who called it "fixed
air" - it won't support combustion or animal life.
At pressure nitrogen acts as an anaesthetic. In water each 10 metres
of depth increases pressure by about 1 atmosphere, so divers cannot go
below 30m breathing ordinary compressed air. Also, if divers surface too
quickly they can get the "bends" - nitrogen bubbles forming
in the blood.
However nitrogen is essential for life and is present in all living cells
(eg, in DNA), amino acids, chlorophyll in plants, etc.
A Christian scientist in the USA, George Washington Carver, refused to
believe that God would create anything useless, as the peanut was then
thought to be. So he kept working with peanuts until he discovered that
peanut plants (and all legumes) have bacteria that can fix nitrogen in
the soil. (It has since been discovered the bacteria have the nitrogenase
enzyme that combines gaseous nitrogen with hydrogen to produce ammonia.)
And not content with that, he kept going and discovered more uses for
peanuts. Encyclopedia Britannica says:
He ultimately developed 300 derivative products from peanuts - among
them cheese, milk, coffee, flour, ink, dyes, plastics, wood stains,
soap, linoleum, medicinal oils, and cosmetics - and 118 from sweet potatoes,
including flour, vinegar, molasses, rubber, ink, a synthetic rubber,
and postage stamp glue.
He improved peanut horticulture so much that he is considered by many
to be the father of the peanut industry. More about peanut butter can
be read on the Aqualab Origin
of Peanut Butter page.
Nitrous
oxide (N2O or laughing gas), is used as an anaesthetic,
a propellent in whipped cream, and also has a
use in car engines, allowng more fuel to be burned, giving greater
power. The three ways it does this are (1) by breaking down at high temperatures
to provide more oxygen, (2) by liquid N2O evaporating it cools
the intake air - cooler air equals denser air - which means the compression
stroke is easier, and (3) forming N2 gas on the power stroke,
releasing lots of energy - see explosives below. It's not all fun and
games, though. From Wikipedia:
One of the major problems of using nitrous oxide in a reciprocating
engine is that it can produce enough power to destroy the engine. Power
increases of 100-300% are possible, and unless the mechanical structure
of the engine is reinforced, most engines would not survive this kind
of operation.
Nitric
oxide (NO) and nitrogen
dioxide (NO2) are very toxic and are bad pollutants mostly
produced by car exhaust. NO is short lived and reacts rapidly in air to
make NO2 which has a brown colour and has "a characteristic
sharp, biting odour." Concentrations as low as 4 ppm can anaesthetise
the nose.
Air glow is a term for light emitted from the upper layers of
the atmosphere of Earth, or of another planet. The glow is generally bluish
in color. One mechanism that produces air glow occurs when an atom of
nitrogen combines with an atom of oxygen to form a molecule of nitric
oxide (NO). In the process a photon is emitted. This photon may have any
of several different wavelengths characteristic of nitric oxide molecules.
The free atoms are available for this process because molecules of nitrogen
(N2) and oxygen (O2) are dissociated by solar energy
in the upper reaches of the atmosphere, and may encounter each other to
form NO.
For Nitrogen and Explosives see Chapter 13 under Explosives.
Links
For more information about how common the constituent air gases are see
this
page.
|

