Chapter 5: Electricity to
the Rescue
static electricity | AC
vs DC | electrolysis | electroplating
| ancient battery
Static Electricity
Electricity is a flow of electrons. Because the word "static"
means "not moving", static electricity is an electric charge
that is built up, but is not an electric current. The zap/spark it gives
is the sudden discharge of that charge.
Static electricity is normally quite safe because even though the voltage
may be several thousand volts the current is very low. One way to work
out how much voltage is generated is to measure the length of the spark
created. Air breaks down at about 1,000 to 3,000 V/mm, depending on humidity,
temperature, etc, so if a spark between two objects is 2 mm long there's
2,000 to 6,000 volts difference between them.
Static electricity is generated when two objects are rubbed together.
The further apart the objects are on the triboelectric
series the larger the charge is likely to be (as a generalisation).
"Tribos" is Greek for "rubbing" while "electron"
is Greek for amber, a material that is good for generating a charge when
rubbed with rabbit fur (for example). The further apart on the list the
objects are, the more easily a large charge can be built up - for example,
dry skin rubbed with a PVC pipe. They're at opposite ends of the series,
so the pipe should be easily charged for some good hair-raising experiences.
See the Van de Graaff picture
page for pictures of a Van
de Graaff generator in action.
Mythbusters investigated how static electricity can cause car fires at
petrol stations. The worst thing to do is to return to your car while
filling it, then get out without touching the door (or anything else),
then grab the pump nozzle (handle). This is quite likely to cause a large
spark between you hand and the pump nozzle, possibly lighting petrol fumes.
Mobile phones do not cause petrol station fires.
AC vs DC
AC stands for alternating current. In a circuit the electricity keeps
changing direction. Our mains power is 230 volts, 50 hertz AC (230 V,
50 Hz), which means that the current changes direction 100 times every
second, flowing in one direction for 1/100 second
then in the other direction for 1/100 second, and
so takes 1/50 second to complete one full cycle.
50 hertz means 50 cycles per second.
Mains power hurts (hertz) because the voltage is high, and so any current
will also be high for any particular object like a human.
DC stands for direct current. In a circuit the electrons are always moving
around the circuit in the same direction.
Electrolysis
 Electricity
can be used to break molecules apart, and is an efficient and inexpensive
way of making aluminium, hydrogen, etc. Sodium can be made by electrolysis
of table salt, sodium chloride (NaCl). Electricity
can be used to break molecules apart, and is an efficient and inexpensive
way of making aluminium, hydrogen, etc. Sodium can be made by electrolysis
of table salt, sodium chloride (NaCl).
Nuclear submarines are able to generate breathing oxygen from the water
around them, so can remain underwater for as long as their fuel lasts
(months).
The reaction to break water (H20) into hydrogen and oxygen
gas is the opposite to that when hydrogen burns. Instead of energy being
given out in the combustion reaction, energy is put in by the electricity.
2H2 + O2 → 2H2O +
heat
2H2O + energy → 2H2 + O2
Direct current is used, so one electrode in a solution will always be
positive (anode) and the other will be negative (cathode). Each electrode
attracts ions which are of the opposite charge. If we rewrite the equation
to show the ions (charged atoms) involved we get:
4H+ + 2O- + energy→ 2H2 + O2
So the hydrogen ions will be attracted to the negatively charged electrode
- the cathode. If we did electrolysis in a cup of water the hydrogen could
be collected by holding a test tube over the cathode, since hydrogen is
lighter than air and any air will be pushed out the bottom of the test
tube by the "floating" hydrogen. To test that we do have hydrogen
we would use the pop test.
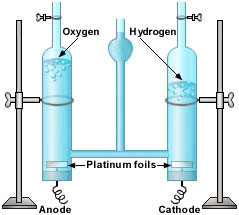
In the Hoffman apparatus pictured, while the taps are turned off the
gases collect in the top of the apparatus, pushing the water levels down.
Note that twice as much hydrogen is formed.
Electroplating
 Electricity
can be used to electroplate
conductive (normally metal) items. Electricity
can be used to electroplate
conductive (normally metal) items.
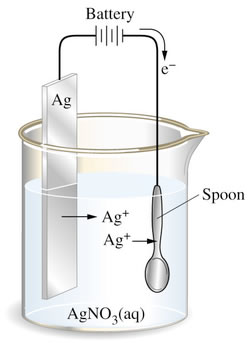
For an item to be silver plated, as illustrated at right, a solution
of silver nitrate is used with a silver anode to supply the silver.
The item itself forms the cathode, which is why the item needs to
be conductive.
The longer the item is left the thicker the plating, but Wikipedia
points out that "considerable skill and craft-technique is
required to ensure an evenly-coated finished product."
Advantages of electroplating audio connectors with gold include:
- Good conductivity.
- Doesn't tarnish.
- Inexpensive compared to solid gold (which would be soft).
|
Ancient battery
About a dozen clay pots - apparently batteries - were found near
Baghdad in the 1930s and date from around AD 225. That's 1,575 years
before modern batteries were invented by Alessandro
Volta in 1800.
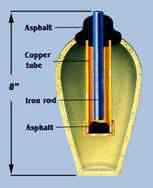 Each
pot is about 15cm high and contains a cylinder made of sheet copper.
The edge of the copper cylinder is soldered with a 60-40 lead-tin
alloy similar to today's solder. The bottom of the cylinder is capped
with a crimped-in copper disk and was sealed with bitumen or asphalt.
Another insulating layer of asphalt sealed the top and also held
in place an iron rod suspended into the center of the copper cylinder.
The rod showed signs of corrosion by an acid, which was probably
vinegar or grape juice. Each
pot is about 15cm high and contains a cylinder made of sheet copper.
The edge of the copper cylinder is soldered with a 60-40 lead-tin
alloy similar to today's solder. The bottom of the cylinder is capped
with a crimped-in copper disk and was sealed with bitumen or asphalt.
Another insulating layer of asphalt sealed the top and also held
in place an iron rod suspended into the center of the copper cylinder.
The rod showed signs of corrosion by an acid, which was probably
vinegar or grape juice.
While many (most?) archeologists apparently believe the pots were not used as batteries, no one is
quite sure what they were used for. If they were batteries, iron and copper cannot produce
much voltage (0.78 V), and because the surface area of the iron and copper
is not very high, not much current would have been produced. Some
ideas of what they may have been used for include electroplating,
electric acupuncture, and making idols tingle when touched, to give
the impression there's something magical about them.
 The
archeologist who discovered the batteries also found copper vases
plated with silver in the Baghdad Museum, which are believed to
date back to at least 2500 BC. The
archeologist who discovered the batteries also found copper vases
plated with silver in the Baghdad Museum, which are believed to
date back to at least 2500 BC.
In 1940, Willard F.M. Gray, an engineer at the General Electric
High Volatage Laboratory in Pittsfield, Massachusetts, USA, made
a replica of the battery. Using copper sulphate solution, it generated
about half a volt of electricity.
In the 1970s German Egyptologist Arne Eggebrecht built a replica
of the Baghdad battery and filled it with freshly pressed grape
juice, generating a claimed 0.87 V. He used current from the battery
to electroplate a silver statuette with gold.
Mythbusters also tested the batteries in episode
29 and came to the conclusion that it's plausible they were
used for electroplating, as they successfully electroplated a small
medallion. Acupuncture was also deemed possible. They also connected
their series of 10 batteries to an electric fence controller, providing
10,000 volts to some figurines, and had "fun" zapping each other.
For more information see news.bbc.co.uk/2/hi/science/nature/2804257.stm. |
|

