Chapter 7: Sunlight Shows
the Way
refraction | spectral
lines | new elements | astronomy
Refraction
When light passes from one medium to another of a different optical density
the light gets bent. For example, when light passes from air to glass
or glass to air. This bending of light at the surface between the two
optical densities is called refraction. It is not the same as reflection,
which is when light bounces off a surface.
Different wavelengths, or colours, of light get bent a different amount,
resulting in a spectrum. A rainbow is a good example of a naturally
occuring spectrum.
A prism is a block of glass (or whatever) with two of its faces
at an angle to each other. When white light passes through a prism the
white light entering is separated into a spectrum, then separated more
when it leaves because of the different angle of the second prism surface.
The same principle applies to raindrops, resulting in rainbows.
While pretty to see, it can be quite annoying for people who wear glasses,
because everything on the edge of their lenses has a spectrum.
In the picture below we have some light reflected away by the first surface
(the beam at top right) and some reflected inside the prism by the second
surface (it comes out at the bottom of the prism). The most interesting
bit is the refracted light which comes out as a spectrum on the left.

Aside: After the chemistry lesson I went home and washed my car, and
noticed that with the hose set on a wide spray held just right I could
see a whole double rainbow at once (except for the bottom where my shadow
was). I got a little wet though.
Spectral lines
Refraction is important because when substances are heated enough they
emit light, and this applies whether it's the Sun or a light bulb filament. But when they're
heated in the right way (eg, as a gas or plasma rather than as a solid)
the light produced is not a smooth spectrum containing all colours evenly.
The light will instead consist of spectral
lines, which show up as bright or dark lines across the spectrum.
 Spectral
lines can be either absorption lines (dark lines) or emission
lines (bright lines), depending on whether they are dimmer or
brighter than the "background" light. Spectral
lines can be either absorption lines (dark lines) or emission
lines (bright lines), depending on whether they are dimmer or
brighter than the "background" light.
Absorption lines are often only visible when the spectrum is spread
out wide enough. Even though we can't normally see it with our eyes,
sunlight reaching earth's surface has hundreds of absorption lines,
where some colours, in very narrow colour bands, get absorbed by
different elements, either at the sun itself or in our atmosphere.
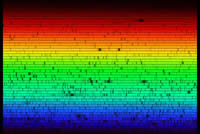
Click the graphic at right for a larger version of the sun's spectrum.
This spectrum is so spread out that the absorption lines can be
seen. Each row in the spectrum continues on to the next row down,
a bit like lines of text in a book. |
Each element has its own set of spectral lines, and these can be very
different.
For example, the emission spectrum of hydrogen (H) with a strong red
spectral line at 656.28 nanometres and blue at 486.13 nanometres:

Compare that to the emission spectrum of iron (Fe):

Sodium has a distinctive pair of spectral lines in the orange/yellow
part of the spectrum. When low pressure sodium vapour lamps are first
turned on they are a red colour because they are acting as neon lamps
(neon has strong red spectral lines) but as the lamp warms up the sodium
vapourises and the sodium starts producing light in the distinctive orange/yellow
colour that the lamps (and sodium itself) are known for. In the sun's
spectrum (thumbnail at above right), sodium's pair of spectral lines appear as easily-spotted
absorption lines.
Absorption lines in the Sun's spectrum were first discovered in 1802
by an English chemist named William Hyde Wollaston. Wikipedia: In 1814,
Joseph von Fraunhofer independently rediscovered the lines and began a
systematic study and careful measurement of the wavelength of these features.
In all, he mapped over 570 lines, and designated the principal features
with the letters A through K, and weaker lines with other letters.
These spectral lines form a set known as Fraunhofer
lines.
The lines he designated A and B were prominant absorption lines in the
red part of the spectrum. These are from oxygen in our atmosphere.
He gave C to the (red) hydrogen alpha line, while F he gave to the blue
hydrogen beta line (see above emission spectrum for H).
Sodium's characteristic double yellow band are known as the Sodium D-lines
at 588.9950 and 589.5924 nanometers.
E was appointed for one of iron's lines, the green 526.96nm line.
New elements
Several elements were discovered by spectroscopy, the first being caesium
(Cs) in 1860 (note the spelling – caesium has an a in a funny place).
Caesium was named after its two bright blue spectral lines 22 years before
it was actually physically isolated. Its name is slightly ironic because
it comes from Latin caesius meaning "sky blue" or "light
blue" but in 1882 when the first caesium metal was produced, it was
found the metal is actually silvery gold in colour (it's one of only three
coloured metals). Caesium is very reactive and will explode even in
cold water or with ice.
Helium
(He) was discovered in the sun 37 years before it was discovered in usable
quantities on earth (in natural gas). Helium means "from the Sun",
from the Greek word for Sun, helios. Helium is special in a lot
of ways, including that it will remain a liquid down to absolute zero
at normal pressures. At pressure, helium can solidify and is colourless
and almost invisible.
Thallium
(positioned between mercury and lead in the periodic table), cerium
and others were also discovered using spectroscopy.
For a while astronomers thought they had found a new element in nebulae
that had emission lines like no other element known. They called it nebulium
but in 1927 nebulium was found to be oxygen. Because it was much much
thinner in the nebulae than it is on Earth the oxygen was behaving quite
differently and making some quite different emission lines.
Astronomy
 Planets,
Asteroids, Comets, etc. These solar system bodies rely on reflecting
the Sun's light, and their materials cause absorption lines in the
light reflected. By comparing the sun's spectral lines with the
extra spectral lines of the object, we can get some idea of what
the object is made of. Wikipedia: Asteroids can be classified
into three main types, according to their spectra: the C-types are
made of carbonaceous material, S-types consist mainly of silicates,
and M-types are 'metallic'. C- and S-type asteroids are the most
common. Similarly, we can see that comets are basically dirty
iceballs. For more info see astronomical
spectroscopy or for some photographs of comets see the Comets page. Planets,
Asteroids, Comets, etc. These solar system bodies rely on reflecting
the Sun's light, and their materials cause absorption lines in the
light reflected. By comparing the sun's spectral lines with the
extra spectral lines of the object, we can get some idea of what
the object is made of. Wikipedia: Asteroids can be classified
into three main types, according to their spectra: the C-types are
made of carbonaceous material, S-types consist mainly of silicates,
and M-types are 'metallic'. C- and S-type asteroids are the most
common. Similarly, we can see that comets are basically dirty
iceballs. For more info see astronomical
spectroscopy or for some photographs of comets see the Comets page.
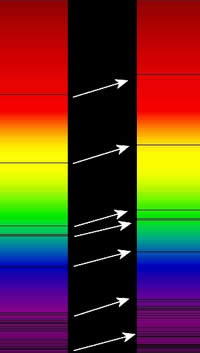
Motion and redshift. If a distant galaxy is moving toward
or away from us the light waves will be shrunk or stretched, a little
bit like the horn on a car or train, or the siren on an ambulance
or police car sounding higher pitched when it is coming towards
us and lower pitched when it is going away from us. This will mean
the spectral lines will be moved slightly from where we would expect
to see them – toward the blue if an object is coming toward us and
toward the red if the object is heading away from us.
Because of this we know that Andromeda Galaxy is moving toward
us at about 100-140 km/s – because its spectral lines have a blueshift.
The largest blueshift of any "nearby" star belongs to
Woolley 9722, which is heading toward us at 260 km/s. Thankfully
it's 78.2 light-years away.
Most galaxies have their spectral lines redshifted – example in
the pic at right. |
|

